Centre for Experimental Cardiooncology
The Centre for Experimental Cardiooncology (CKOD) is a university-wide unit on the map of initiatives supported under the Excellence Initiative – Research University program. It was established in 2025 to optimize the use of MUG’s scientific and research potential in the topics of cardiology, oncology and on the frontier where these fields meet. The establishment of the CKOD is a direct result of the collaboration between researchers from experimental and clinical sciences, fitting into all the Priority Research Areas of MUG, i.e.: Oncology, Cardiology and Cardiovascular Medicine, Biochemistry, Genetics and Molecular Biology. The unit carries out translational projects (from bench to bedside and back again), offering the real prospect of implementing diagnostic and therapeutic solutions developed using unique experimental models and advanced research techniques in standard clinical practice.
An important element of the unit’s current work is the integration of the interdisciplinary community of researchers from the Medical University of Gdańsk and the University Clinical Center in Gdańsk with scientists from the Jagiellonian University, the Ludwik Hirszfeld Institute of Immunology and Experimental Therapy of the Polish Academy of Sciences, Institute of Animal Reproduction and Food Research of the Polish Academy of Sciences in Olsztyn, Maria Skłodowska-Curie National Institute of Oncology, National Research Institute,Warsaw University of Technology, Center for Postgraduate Education in Warsaw and the National Institute of Cardiology – National Research Institute, among others. The goal of this broad collaboration is to develop state-of-the-art diagnostics and therapeutics for cardiovascular diseases with a special focus on conditions involving endothelial dysfunction, which can be triggered by neoplastic disorders, cancer therapies and coexisting cardiovascular risk. In addition, CKOD conducts research on the molecular and cellular reprogramming mechanisms of heart conduction induced by radiation therapy.
OUR POTENTIAL AND RANGE OF SERVICES
The overarching goal of CKOD’s activities is to develop innovative diagnostic and therapeutic solutions to current clinical challenges in cardiology, oncology and at the interface of these fields. We conduct translational research using unique experimental models and advanced research techniques.
CKOD’s main areas of activity include:
- Application of cellular and animal models, as well as live organotypic specimens of the human heart and blood vessels ex vivo in experimental studies;
- Developing new diagnostic strategies by searching for biomarkers of early cardiovascular damage in neoplastic disorders and during cancer therapy;
- Searching for new therapeutic targets in cardiovascular pathologies in oncology patients
- Interdisciplinary research projects based on collaboration with experts within IDUB Priority Research Areas and universities affiliated with the Fahrenheit Union of Universities
Research facilities and available equipment:
As a part of its activities, CKOD researches are conducted in 6 laboratories:
1. Laboratory of intravital organotypic specimens of the heart and vessels
2. Laboratory for isolation and culture of primary cells and cell lines
3. Laboratory for imaging of tissues, organotypic specimens, organoids and cell cultures
4. Laboratory of cell and tissue metabolism
5. Laboratory for irradiation of materials, cells and tissues
6. In vivo research laboratory
1. Laboratory of intravital organotypic specimens
- Oscillating microtome with cooling for slicing live tissue under oxygenated isolation buffer conditions
- Incubator for culture of organotypic specimens (thickness: 100 – 300 μm) with controlled carbon dioxide atmosphere
- Isolation and culture system compatible with imaging and metabolic analysis
- System for recording action potentials in cardiac slices, Multielectrode Array – MEA
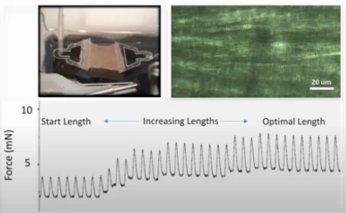
- System for culturing heart specimens under electric field stimulation (MyoDish In Vitro System)
- Ex vivo vessel isolation system
- An eight-channel vascular disc bath kit (Danish Myo Technology – DMT) with an advanced semi-automatic flushing system, designed to studying the endothelium-dependent and -independent responses of isolated vascular discs removed during routine invasive procedures.
2. Laboratory for isolation and culture of primary cells and cell lines
- Cell culture incubator with controlled atmosphere of carbon dioxide and oxygen
- Laminar chambers with vertical and horizontal air flow
- Inverted light microscope
- High-speed refrigerated laboratory centrifuges
- Microhematocrit centrifuge
- Microcentrifuge
3. Laboratory for imaging of tissues, organotypic specimens, organoids and cell cultures
- Zeiss Axio Observer 7 inverted fluorescence microscope, equipped with incubation chamber with dual-channel gas mixer (with the ability to adjust oxygen level in the chamber) and temperature control, Axiocam 105 color camera, Axiocam 305 digital monochrome camera, full x/y/z automation, autofocus, ApoTome.2 structured fluorescence illumination system, EC Plan-Neofluar lens set (10x, 20x, 40x, 62x), HXP 120 light source, 2 sets of filters: 1. DAPI, FITC, TRITC, Cy5 and 2. CFP, YFP, Hc Red, Nomarski DIC contrast, and Zeiss ZEN 2.6 pro Hardware software package
- Orbitrap Exploris 480 mass spectrometer interfacing with AP-MALDI UHR mass imaging source (MassTech)
4. Laboratory of cell and tissue metabolism
- Nexera LC 2040 (Shimadzu) and Nexera LC40 (Shimadzu) liquid chromatographs
- Orbitrap Exploris 480 mass spectrometer interfacing with Vanquish liquid chromatograph or Ultimate 3000 nanochromatograph (ThermoFisher)
- MicroTOF-Q2 mass spectrometer (Bruker) cooperating with Ultimate 3000 chromatograph (ThermoFisher) or Ultimate 3000 nanochromatograph
- Quantum TSQ Vantage EMR mass spectrometer cooperating with Surveyor chromatograph or Ultimate 3000 nanochromatograph (ThermoFisher).
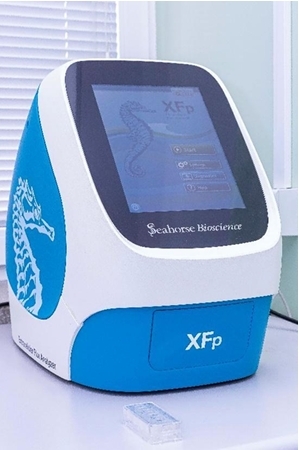
- Seahorse XFp Metabolic Analyzer (Seahorse BioScience), which enables measurements of oxygen consumption rates, reflecting mitochondrial respiration and extracellular acidification rates, reflecting glycolysis in live cells, 3D cell models and tissue sections. The rate of oxygen consumption can also be measured in isolated mitochondria or permeabilized cells.
- The XL-180 automatic biochemical analyzer (Erba, Meinheim) with an open system is designed for spectrophotometric and immunoturbidimetric measurements (in the 340-700 nm wavelength range). The analyzer has an extensive test menu for biochemical determinations in blood serum, plasma and urine. The device has an extensive quality control program, and can perform up to 180 tests per hour. The small volume of reaction mixture (180 µL) dispensed with a precision sapphire syringe allows using a small amount of biological material.
5. Laboratory for irradiation of materials, cells and tissues
- Varian Truebeam HD-type accelerator using 6MV accelerating voltage
- Plexiglas phantoms with a set of Farmer-type chambers for precise measurements of weekly doses.
- Quickcheck device, used for daily monitoring of accelerator stability.
- MPC phantom, enabling daily inspection of full device geometry.
- Semiflex 3D chambers, designed for water phantom measurements, and a Diamond chamber, perfect for small-field measurements in phantom conditions.
- Octavius 4D PTW phantom and solid-state array providing the ability to verify irradiation plans for both conventionally fractionated radiotherapy and stereotactic techniques.
- A plan verification system using an electronic picture detector (EPID) and Gafchromic™ films, supported by a dedicated scanner and film analysis software.
- Mobius independent dose calculation system for independent verification of dose distribution, independent of the radiotherapy planning system.
- Acuros and AAA algorithms, allowing for more precise dose calculations, especially in the presence of high-density materials such as titanium implants.
- IMAR imaging system on CT, supporting precise localization of cardiac structures with consideration of different-density materials.
6. In vivo research laboratory
- Rat and mouse models of cardiovascular pathologies, including hypertension, dyslipidemia, atherosclerosis, cardiac insufficiency and arrhythmias
- Mouse models of neoplastic disorders, including breast cancer, lung cancer, pleural mesothelioma and melanoma.
- Ultrasound and ECHO machine-Vero 1100 Imaging System
- ECG device
- Inhalation anesthesia system
- Langerdorf perfusion system
- Respirator for laboratory animals
CKOD’s activities are aimed at:
- Reinforcing the collaboration between researchers in experimental and clinical sciences
- Increasing the potential for translational projects that incorporate preclinical research in cardiology, oncology and at the intersection of these fields
- Reducing the incidence of cardiovascular complications in oncology-treated patients, which directly translates into better quality and life expectancy.
- Early diagnostics and precise monitoring of cardiovascular status during and after cancer therapy by implementing modern diagnostic tools.
- Personalizing treatment – better matching the intensity and type of therapy to the individual profile of the patient, taking the cardio and vasculotoxicity risk into account.
- Drug repurposing, i.e., demonstrating the beneficial effects of known molecules in the face of prevention and treatment of cardiovascular conditions in oncology patients.
- Improving the efficacy of radiotherapy and chemotherapy by optimizing doses and treatment regimens that minimize myocardial and vascular damage.
RANGE OF SERVICES
The Centre provides services to external entities. The unit will perform analyses according to individual orders with customization to meet the specific needs of contractors. The cost of the service is priced individually.
The research team includes:
Subject matter managers and project leaders:
- Dr. Habil. Barbara Kutryb-Zając, supervision of laboratories and experimental models
- Bartłomiej Tomasik, Ph.D., supervision of the irradiation laboratory and electrophysiology experiments using multi-electrode arrays (MEA)
Laboratory supervisors:
- Alicja Braczko, Ph.D. supervisor of the Cell and Tissue Metabolism Laboratory
- Joanna Kamińska, Ph.D. Eng., supervisor of the Laboratory of Irradiation of Materials, Cells and Tissues
- Marika Frańczak, M.A., supervisor of the Laboratory of Isolation and Culture of Primary Cells and Cell Lines
- Ada Kawecka, M.A., supervisor of the Living Organotypic Specimens Laboratory and the Imaging Laboratory
- Klaudia Stawarska, M.A., supervisor of the Living Organotypic Specimens Laboratory and the Imaging Laboratory
- Iga Walczak, M.A., supervisor of the Laboratory of Isolation and Culture of Primary Cells and Cell Lines
- Krzysztof Urbanowicz, Eng. M., supervisor of the Laboratory of Cell and Tissue Metabolism
Scientific achievements of the team:
van der Pol LHG, Blanck O, Grehn M, Blazek T, Knybel L, Balgobind BV, Verhoeff JJC, Miszczyk M, Blamek S, Reichl S, Andratschke N, Mehrhof F, Boda-Heggemann J, Tomasik B, Mandija S, Fast MF. Auto-contouring of cardiac substructures for Stereotactic arrhythmia radioablation (STAR): A STOPSTORM.eu consortium study. Radiother Oncol. 2024 Nov 1;202:110610.
Zheng XF, Sarkar A, Lotana H, Syed A, Nguyen H, Ivey RG, Kennedy JJ, Whiteaker JR, Tomasik B, Huang K, Li F, D’Andrea AD, Paulovich AG, Shah K, Spektor A, Chowdhury D. CDK5-cyclin B1 regulates mitotic fidelity. Nature. 2024 Sep;633(8031):932-940.
Zabielska-Kaczorowska M.A.,Stawarska K.,Kawecka A., Urbanowicz K., Smolenski R.T., Kutryb-Zajac B. Nucleotide depletion in hypoxia experimental models of mouse myocardial slices. Nucleosides, Nucleotides & Nucleic Acids, 2024, 43(8), 770–782.
Swift ML, Zhou R, Syed A, Moreau LA, Tomasik B, Tainer JA, Konstantinopoulos PA, D’Andrea AD, He YJ, Chowdhury D. Dynamics of the DYNLL1-MRE11 complex regulate DNA end resection and recruitment of Shieldin to DSBs. Nat Struct Mol Biol. 2023 Oct;30(10):1456-1467. doi: 10.1038/s41594-023-01074-9.
Miszczyk M, Sajdok M, Bednarek J, Latusek T, Wojakowski W, Tomasik B, Wita K, Jadczyk T, Kurzelowski R, Drzewiecka A, Cybulska M, Gardas R, Jarosiński G, Dolla Ł, Grządziel A, Zub K, Bekman A, Kaminiów K, Kozub A, Gołba KS, Blamek S. Stereotactic management of arrhythmia – radiosurgery in treatment of ventricular tachycardia (SMART-VT). Results of a prospective safety trial. Radiother Oncol. 2023 Nov;188:109857.
Chałubińska-Fendler J, Nowicka Z, Dróżdż I, Graczyk Ł, Piotrowski G, Tomasik B, Spych M, Fijuth J, Papis-Ubych A, Kędzierawski P, Kozono D, Fendler W. Radiation-induced circulating microRNAs linked to echocardiography parameters after radiotherapy. Front Oncol. 2023 May 18;13:1150979. doi: 10.3389/fonc.2023.1150979.
Hawryszko M, Sławiński G, Tomasik B, Lewicka E. Cardiac Arrhythmias in Patients Treated for Lung Cancer: A Review. Cancers (Basel). 2023 Dec 6;15(24):5723.
Grehn M, Mandija S, Miszczyk M, Krug D, Tomasik B, Stickney KE, Alcantara P, Alongi F, Anselmino M, Aranda RS, Balgobind BV, Boda-Heggemann J, Boldt LH, Bottoni N, Cvek J, Elicin O, De Ferrari GM, Hassink RJ, Hazelaar C, Hindricks G, Hurkmans C, Iotti C, Jadczyk T, Jiravsky O, Jumeau R, Kristiansen SB, Levis M, López MA, Martí-Almor J, Mehrhof F, Møller DS, Molon G, Ouss A, Peichl P, Plasek J, Postema PG, Quesada A, Reichlin T, Rordorf R, Rudic B, Saguner AM, Ter Bekke RMA, Torrecilla JL, Troost EGC, Vitolo V, Andratschke N, Zeppenfeld K, Blamek S, Fast M, de Panfilis L, Blanck O, Pruvot E, Verhoeff JJC. STereotactic Arrhythmia Radioablation (STAR): the Standardized Treatment and Outcome Platform for Stereotactic Therapy Of Re-entrant tachycardia by a Multidisciplinary consortium (STOPSTORM.eu) and review of current patterns of STAR practice in Europe. Europace. 2023 Apr 15;25(4):1284-1295.
Kutryb-Zajac B., Kawecka A., Nasadiuk K., Braczko A., Stawarska K., Caiazzo E., Koszalka P., Cicala C. Drugs targeting adenosine signaling pathways: a current view. Biomedicine & Pharmacotherapy, 2023, 165, 115184
Fendler W, Tomasik B, Atkins K, Stawiski K, Chałubińska-Fendler J, Kozono D. The clinician’s guide to radiotherapy complications. Pol Arch Intern Med. 2022
Kutryb-Zajac B., Kawecka A., Braczko A., Franczak M., Slominska EM., Giovannoni R., Smolenski RT. CoCl2-mimicked endothelial cell hypoxia induces nucleotide depletion and functional impairment that is reversed by nucleotide precursors. Biomedicines, 2022, 10(7), 1540
Koszalka P., Kutryb-Zajac B., Mierzejewska P., Tomczyk M., Wietrzyk., Serafin P., Smolenski RT., Slominska EM. 4-Pyridone-3-carboxamide-1-β-D-ribonucleoside (4PYR) — A Novel Oncometabolite Modulating Cancer-Endothelial Interactions in Breast Cancer Metastasis. International Journal of Molecular Sciences, 2022, 23(19), 5774
Jedrzejewska A., Kutryb-Zajac B., Krol O., Harasim G., Franczak M., Jablonska P., Slominska EM., Smolenski RT. The decreased serum activity of cytosolic 5’-nucleotidase IA as a potential marker of breast cancer-associated muscle inflammation. Nucleosides Nucleotides Nucleic Acids, 2022, 41(3), 273-284.
Mierzejewska P., Kunc M., Zabielska-Kaczorowska M., Kutryb-Zajac B., Pelikant-Malecka I., Braczko A., Jablonska P., Romaszko P., Koszalka P., Szade J., Smolenski RT, Slominska EM. An unusual nicotinamide derivative, 4-pyridone-3-carboxamide ribonucleoside (4PYR), is a novel endothelial toxin and oncometabolite. Experimental and Molecular Medicine, 2021, 53, 9, 1402-1412
Kutryb-Zajac B., Harasim G., Jedrzejewska A., Krol O., Braczko A., Jablonska P., Mierzejewska P., Zielinski J., Slominska EM. Smolenski RT. Macrophage-Derived Adenosine Deaminase 2 Correlates with M2 Macrophage Phenotype in Triple Negative Breast Cancer. Int. J. Mol. Sci. 2021, 22(7), 3764;
Mateuszuk Ł., Campagna R., Kutryb-Zając B., Kuś K.l, Słomińska EM., Smoleński RT, Chlopicki S. Reversal of endothelial dysfunction by nicotinamide mononucleotide via extracellular conversion to nicotinamide riboside. Biochem. Pharmacol. 2020 : vol. 178, art. ID 114019, s. 1-11.
Chalubinska-Fendler J, Graczyk L, Piotrowski G, Wyka K, Nowicka Z, Tomasik B, Fijuth J, Kozono D, Fendler W. Lipopolysaccharide-Binding Protein Is an Early Biomarker of Cardiac Function After Radiation Therapy for Breast Cancer. Int J Radiat Oncol Biol Phys. 2019 Aug 1;104(5):1074-1083. doi: 10.1016/j.ijrobp.2019.04.002.
CONTACT

Dr. Habil. Barbara Kutryb-Zając, Assoc. Prof.
Department of Biochemistry
Medical University of Gdańsk
Phone 58 349 14 60
Mail barbara.kutryb-zajac@gumed.edu.pl

Bartłomiej Tomasik, Ph.D.
Department of Oncology & Radiotherapy
Medical University of Gdańsk
Phone 58 584 45 60
Mail bartlomiej.tomasik@gumed.edu.pl
photo Paweł Sudara/MUG & private archive