Laboratory of Intravital Imaging of Cells, Tissues and Organoids
Department of Histology
Faculty of Medicine
ABOUT US
Two laboratories from the Department of Histology and the Department of Biochemistry of the Faculty of Medicine at the Medical University of Gdansk collaborate as a part of the core facility. Both are equipped with fluorescence inverted microscopes with incubation chambers for long-term observations of cells (2D and 3D cultures), spheroids, explants and organoids. The research can be carried out in both aerobic and oxygen-limited environments.
The laboratory has two unique culture systems based on microfluidic technology. The first, Miconit’s “Organ-on-a-chip”, allows continuous observation of cells under a microscope. The second system, MIVO (Multi In Vitro Organ System) from REACT4LIFE, allows larger spheroids, organoids and explants to be cultured under microfluidic conditions. This system works with an incubator and offers microscopic observation.
The laboratory is equipped with a range of 2D and 3D dynamic image presentation and modeling software, i.e.: celSens Dimension Desktop 4.3 with 2D deconvolution, Nearest Neighbor, Wiener, co-location analysis, and Olympus Deep-Learning Technology’s TruAI™ machine learning analysis platform (Olympus, Japan).
Some capabilities offered by these systems include:
- Studying of intercellular interactions
- Analysis of cell mobility, transmigration across the endothelial barrier, infiltration into organoids and tissues by other cell types (e.g., a model of tumor infiltration by lymphoid cells/cells from secondary foci – metastases)
- Assessment of cell viability and overall condition supported by biochemical tests
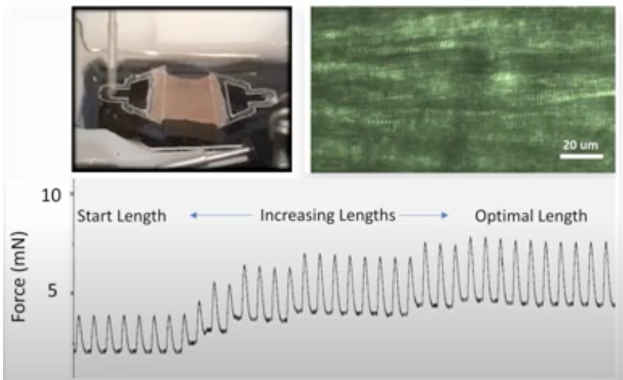
- Assessment of cellular function, such as analysis of nitric oxide production by vascular endothelium, analysis of myocyte contractility by assessing intracellular calcium levels
- Tracking of moving intracellular and extracellular proteins, analysis of their externalization to the cell membrane surface
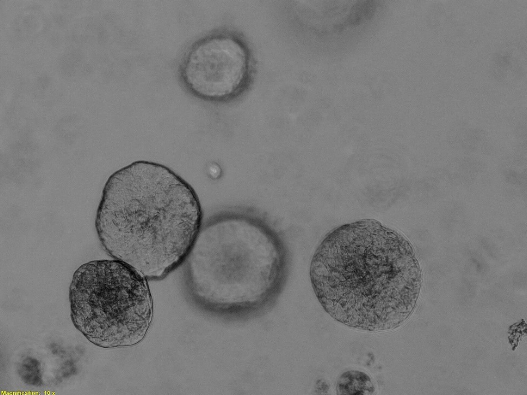
- Developing in vitro 3D models and intravital observation (e.g., of mitochondrial networks, intracellular vesicles)
- Cytotoxicity and drug metabolism tests
- Endothelial shear stress tests
- Culture, observation and stimulation of organotypic section specimens:
- human specimens extracted during surgical procedures, such as from insufficient heart muscle or neoplastic tumors
- from animal experimental models (including brain, heart, lung, kidney)
- Analysis of tissue and organoid ultrastructure, such as visualization of hair follicles or blood vessels
OUR POTENTIAL
Laboratory of intravital organoid studies
Olympus IX83 inverted fluorescence microscope
The microscope is equipped with:
- Incubation chamber with dual-channel gas mixer (with the ability to adjust oxygen level in the chamber) and temperature adjustment
- Color CMOS digital camera
- Ultra-fast monochrome camera (100 fps at full resolution)
- Full automation in x/y/z planes
- Autofocus function
- Contrast-phase lens set and plan-apochromatic 1.25x, 10x, 20x, as well as immersion 60x lenses
- Light source for fluorescence observation: Set of sixteen LED items –
- An eight-position automated carousel for fluorescent filters in the form of a cassette, easily ejected from the microscope. Installed filters: DAPI, FITC, TRITC, Cy5.
- Polarizer
- A software package with full control of microscope functions and image analysis, including a multi-well plate navigator, data analysis and deconvolution module
- Image analysis workstation
Cell and tissue culture laboratory
Zeiss Axio Observer 7 fluorescence inverted microscope
The microscope is equipped with:
- Incubation chamber with dual-channel gas mixer (with the ability to adjust oxygen level in the chamber) and temperature adjustment
- M12 S1 heating insert (D) for 6-, 12-, 24-, 48- and 96-well plates
- Slide and culture dish table adapters
- Axiocam 105 color camera, USB 3.0 PCIe x1 interface, 1 CMOS, 2560 (H) x 1920 (V) = 5.0 Megapixel
- Axiocam 305 mono digital camera (D) USB 1 3.0 PCIe x1 interface, USB 3.0 cable 3 m, 5.07 Mega pixel
- 60N-C 2/3’’ 0.63x video adapter
- Full automation in x/y/z planes
- Autofocus function
- ApoTome.2 fluorescent structured lighting system
- EC Plan-Neofluar contrast-enhanced fluorescence lens kit, adapted for observation with ApoTome.2 (10x, 20x, 40x, 62x)
- Light source for fluorescence observation: Illuminator Kit (D) HXP 120
- A six-position automated carousel for fluorescent filters. Installed 2 sets of filters:
- DAPI, FITC, TRITC, Cy5
- CFP, YFP, Hc Red
- Nomarski DIC contrast
- Zeiss ZEN 2.6 pro Hardware software package with full control of microscope functions and image analysis, along with a multi-well plate navigator, data analysis and deconvolution module
- Image analysis workstation
System for obtaining organotypic section specimens and their culture under long-term incubation conditions
The system is equipped with:
- Oscillating microtome with cooling, enabling tissue slicing under oxygenated isolation buffer conditions
- Incubator for the culture of organotypic section specimens (100 – 300 mm thick) with controlled carbon dioxide atmosphere and oxygen concentration
- Isolation and culture system, compatible with imaging and metabolic analysis performed in the Biochemical Analysis and Imaging Laboratory
- MEA (Multielectrode Array) system for recording action potentials in cardiac section specimens (available soon)
- System for stimulation of cardiac section specimens in an electric field (available soon)
System MIVO (Multi In Vitro Organ System)
The system consists of a peristaltic pump that allows simultaneous culture of up to 8 organoids under forced flow conditions and any combination of them, enabling studies of interactions between organoids or studies of metabolism and toxicity of compounds or drugs.
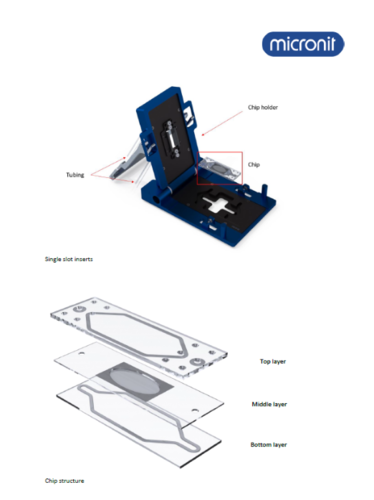
“Organ-on-a-chip” kit from Micronit
This system allows direct and continuous observation of cells or organoids under microfluidic conditions, as it is mounted on the microscope table. This makes it possible to study not only the formation of organoids, but also the direct effect of substances or therapeutics administered using the microfluidic system.
In addition, the laboratory is equipped with:
- Chambers with laminar air flow
- Containers for storing preparations in a liquid nitrogen atmosphere
- Water baths
- Preparative centrifuges, mini-centrifuges and table-top centrifuges
CONTACT
Department of Histology
Prof. Michał Żmijewski
e-mail: mzmijewski@gumed.edu.pl
Department of Biochemistry
Dr. Habil. Barbara Kutryb-Zając