Publication in "Nature" on new mechanisms regulating the cell cycle
5.09.2024

Bartłomiej Tomasik, Ph.D. of the Department of Oncology & Radiotherapy at the Medical University of Gdansk, is a co-author of the article, CDK5–cyclin B1 regulates mitotic fidelity”:https://www.nature.com/articles/s41586-024-07888-x published in Nature. The results of this study, conducted at leading research centers in the United States (Dana-Farber Cancer Institute, Harvard Medical School, Fred Hutchinson Cancer Center), shed new light on the processes that regulate the cell cycle.
– In 2020-2021, as a part of a post-doctoral fellowship carried out thanks to, e.g., the support of the Polish National Agency for Academic Exchange and Prof. Walczak, I worked in the Division of Radiation and Genome Stability, Department of Radiation Oncology, Dana-Farber Cancer Institute, Harvard Medical School. Since then, I have been working closely with two teams headed by Prof. Dipanjan Chowdhury and Prof. Alexander Spektor – said Bartłomiej Tomasik, Ph.D. – The foregoing publication is the result of collaboration between the two teams. My participation in the project focused mainly on experiments related to the evaluation of changes in phosphorylation of proteins involved in mitosis in response to CDK5 inhibition.
Key regulators of the cell cycle are cyclin-dependent kinases (CDKs) and their activators, cyclins. For years, CDK5 was thought to be “atypical” due to its activation by non-cyclin proteins (p35 and p39), and its physiological occurrence only in nerve cells that are not subject to division. Because of that, CDK5 was believed to be a non-cell cycle-associated CDK. At the same time, there were reports indicating that CDK5 was present in excess in many neoplastic diseases, and could drive the proliferation of neoplastic cells, yet the way that CDK5 would regulate the proliferation of these cells remained unclear.
The paper entitled CDK5-cyclin B1 regulates mitotic fidelity showed that CDK5 plays a key role in mitosis. In order to achieve this, the researchers replaced native CDK5 with a variant that specifically binds to substances that inhibit or degrade CDK5 before cells enter mitosis. Scientists have demonstrated that cells lacking CDK5 show mitotic spindle disruption, the structure on which duplicated chromosomes move during cell division. As a result, chromosomes cannot be properly distributed to progeny cells, thus resulting in genetically unstable progeny cells and leading to the formation of abnormal nuclear structures, such as micronuclei. Surprisingly, CDK5 was found to act by cooperating with the B1 cyclin, the same mitotic cyclin that interacts with CDK1, a key regulator of mitosis, in all stages of cell division. Thus, the researchers showed that CDK5 is among the canonical kinases that regulate the cell cycle and is essential for maintaining genome stability.
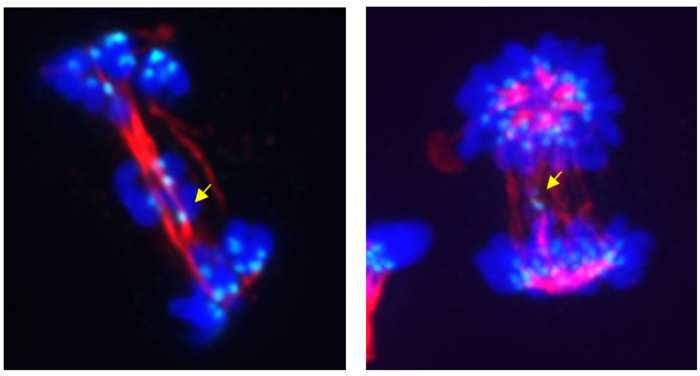
Abnormal cell division due to CDK5 inhibition (left panel) or degradation (right panel) – yellow arrows indicate chromosomes that failed to translocate into progenitor cells during anaphase (lagging chromosomes). The turquoise color represents the anti-centromere antibodies (ACA), which react with one of the centromere-forming proteins, the so-called centromere protein B (CENP-B). The red color represents tubulin, which is part of the division spindle, and the blue color represents Hoechst dye used to stain DNA.
The significance of the discovery
This discovery changes the existing dogma of molecular biology that the CDK1-B1 cyclin complex is the only one essential for all stages of mitosis. The results also suggest that other apparently atypical CDKs may also play a role in cell cycle regulation. Researchers have demonstrated the importance of the CDK5-cyclin B1 complex during physiological cell division, but this complex may play an even greater role in rapidly proliferating neoplastic cells. Indeed, CDK5 is subject to excessive expression and activation in a broad spectrum of neoplasms, and is associated with unfavorable prognosis. This suggests that the development of numerous neoplastic diseases may be dependent on CDK5 levels. Having considered the foregoing, similar to existing therapies targeting CDKs (CDK4/6 inhibitors used to treat breast cancer), CDK5 may constitute an important therapeutic target for cancer treatment in the future.
Bartłomiej Tomasik, Ph.D. is the co-coordinator of the IDUB research team working on biological and physical aspects of ionizing radiation and their translation into clinical practice. The group is actively seeking partners for further scientific activities. Those interested in cooperating can obtain detailed information by e-mail, contacting Dr. Tomasik at bartlomiej.tomasik@gumed.edu.pl.
Graphics private archive