Interdepartmental Laboratory for Analysis of Extracellular Vesicles
Department of Pharmaceutical Microbiology
Department of Toxicology
Faculty of Pharmacy
ABOUT US
The Interdepartmental Laboratory for Analysis of Extracellular Vesicles was established within the framework of the “Initiative of Excellence – Research University” program conducted by the Medical University of Gdansk after establishing cooperation between the Department of Pharmaceutical Microbiology and the Department of Toxicology. The team’s research work focuses on the broad topic of extracellular vesicles. As a part of the laboratory’s work, we conduct isolation and biophysical, biochemical and molecular characterization of extracellular vesicles. We study vesicles of bacterial origin, plant origin and animal organisms, including those from tissue culture.
RESEARCH TOPICS
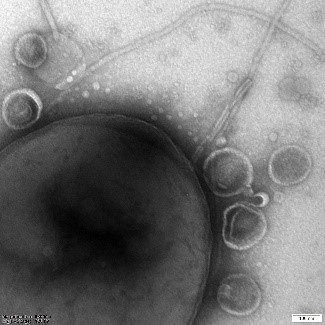
Extracellular vesicles (EVs) are nanostructures secreted by prokaryotic and eukaryotic cells. They can be released naturally (as by-products of cellular metabolism associated with cell wall activity), but also as a response to a stressful condition. The lipophilic membrane properties of EVs allow them to bind to the membranes of target cells and endocytose. They have been detected in human body fluids. In addition to intestinal contents, they are also found in plasma, bile, saliva, ascitic fluid and fluid from abdominal drainage. They are also secreted by neoplastic cells. They can contain various types of biological material, including proteins, nucleic acids, lipids or antibiotics. The vesicle load depends on the growth stage of the parental cells and environmental conditions. It defines their function. Many pathogenic bacteria use EVs to deliver toxic compounds to infected cells. Compounds transported in EVs can also stimulate changes in gene expression. EVs are involved in numerous biological processes, such as cell-to-cell communication, modulation of the immune response and horizontal gene transfer.
B – bacterial cell producing extracellular vesicles
C – plant exosomes and microbubbles seen under cryo-electron microscope
OUR POTENTIAL
Department of Pharmaceutical Microbiology
1. Beckman Coulter Optima™ XPN 100 standing ultracentrifuge with SW 28.1 Ti, SW 70.1 Ti and SW 50.1 Ti rotors
2. CFX Connect Real-Time PCR Detection System Bio-Rad
3. Tecan Infinite M200 Pro microplate reader
Department of Toxicology
1. PACE MDQ plus Sciex capillary electrophoresis device
Optima™ XPN 100 Beckman Coulter ultracentrifuge with SW 28.1 Ti, SW 70.1 Ti and SW 50.1 Ti rotor sets
CFX Connect Real-Time PCR Detection System Bio-RadInfinite M200 Pro Tecan
PACE MDQ plus Sciex capillary electrophoresis device
Beckman Coulter ’s Optima™ XPN 100ultracentrifuge allows centrifugation at speeds of up to 100,000min-¹. The touchscreen can be used both as an information display and a control input for the device. The rotor chamber is made of aluminum and coated with chemically resistant epoxy. The ultracentrifuge meets safety standards for working with biological infectious material.
We have SW 28.1 Ti, SW 70.1 Ti and SW 50.1 Ti rotors that enable ultracentrifugation of a wide range of sample volumes at varying centrifugation speeds.
The PACE MDQ plus capillary electrophoresis system from Sciex, equipped with a PDA and LIF detector, allows assessing the purity of the samples analyzed, as well as to study the heterogeneity of vesicles in the isolate. It also allows determining the vesicle contents of a sample based on comparison with reference vesicles.
Bio-Rad’s CFX Connect Real-Time PCR Detection System thermocycler is a six-channel (five color and one FRET channel) real-time PCR system. It provides sensitive and reliable detection for singplex or multiplex responses. Detects up to five targets at sample volumes of 10 µl. It has a thermal gradient function. CFX Maestro software allows data analysis.
The Tecan Infinite M200 Pro Tecan reader is a multifunctional microplate reader. It offers the ability to perform analysis based on absorbance and fluorescence. Detection is based on monochromators and does not require filters, allowing users to measure absorbance between 230 and 1,000 nm or fluorescence between 300 and 600 nm at 1 nm intervals. The device also enables absorption, excitation and emission scans. It has an injector option.
SCOPE OF SERVICES
- isolation of extracellular vesicles from biological material using ultracentrifugation, precipitation, exclusion chromatography and ultrafiltration techniques
- evaluation of extracellular vesicles in the sample: protein content assay (BCA test)
- biophysical analysis of vesicles: purity analysis of isolates (capillary electrophoresis)
- biochemical characterization of vesicles including measurement of enzymatic activity of intravesicular proteins by fluorometric and colorimetric methods
- molecular characterization including immunodetection of extracellular vesicle markers using Western blot technique
- isolation of mRNA and miRNA from extracellular vesicles and detection of selected biomarkers using RT-qPCR technique
RESEARCH TEAM
The research team includes specialists with years of experience working with extracellular vesicles.
- Dr. Habil. Krzysztof Waleron, Assoc. Prof.
- Dr. Habil. Szymon Dziomba
- Joanna Jońca, Ph.D.
- Jolanta Ficińska-Mazurczyk, Ph.D.
- Aleksandra Steć, M.A.
CURRENT COOPERATION
1. Grant OPUS w konsorcjum z MWB UG GUMed finansowany przez Narodowe Centrum Nauki: Ekologiczna biochemia Pectobacterium – badanie interakcji pomiędzy bakteriami a roślinami. 2019/35/B/NZ9/01973
2. Grant PRELUDIUM finansowany przez Narodowe Centrum Nauki: Ocena czystości i identyfikacja pęcherzyków zewnątrzkomórkowych z wykorzystaniem elektroforezy kapilarnej (nr 2022/45/N/NZ7/01417)
PUBLICATIONS
1. Steć A., Heinz A., Dziomba S. Characterization of extracellular vesicles by capillary zone electrophoresis: A novel concept for characterization of a next-generation drug delivery platform. J Pharm Anal. 2024 May 101004. https://doi.org/10.1016/j.jpha.2024.101004
2. Jonca J, Pirhonen M, Waleron MM, Gawor J, Mrozik A, Smoktunowicz M, et al. Comprehensive phenomic and genomic studies of the species, Pectobacterium cacticida and proposal for reclassification as Alcorniella cacticida comb. nov. Front Plant Sci. 2024 Jan 25;15:1323790. https://doi.org/10.3389/FPLS.2024.1323790/BIBTEX
3. Borowska-Beszta M, Smoktunowicz M, Horoszkiewicz D, Jonca J, Waleron MM, Gawor J, et al. Comparative genomics, pangenomics, and phenomic studies of Pectobacterium betavasculorum strains isolated from sugar beet, potato, sunflower, and artichoke: insights into pathogenicity, virulence determinants, and adaptation to the host plant. Front Plant Sci. 2024 Mar 21;15:1352318. https://doi.org/10.3389/FPLS.2024.1352318/BIBTEX
4. Bleibel L, Dziomba S, Waleron KF, Kowalczyk E, Karbownik MS. Deciphering psychobiotics’ mechanism of action: bacterial extracellular vesicles in the spotlight. Front Microbiol. 2023 Jun 15;14:1211447. https://doi.org/10.3389/FMICB.2023.1211447/BIBTEX
5. Steć A, Targońska M, Karkosińska E, Słowik M, Płoska A, Kalinowski L, et al. Protein overproduction alters exosome secretion in Chinese hamster ovary cells. Anal Bioanal Chem. 2023 Jul 1;415(16):3167–76. https://doi.org/10.1007/S00216-023-04725-4/FIGURES/3
6. Steć A, Chodkowska M, Kasprzyk-Pochopień J, Mielczarek P, Piekoszewski W, Lewczuk B, et al. Isolation of Citrus lemon extracellular vesicles: Development and process control using capillary electrophoresis. Food Chem. 2023 Oct 30;424:136333. https://doi.org/10.1016/J.FOODCHEM.2023.136333
7. Steć A, Jońca J, Waleron K, Waleron M, Płoska A, Kalinowski L, et al. Quality Control of Bacterial Extracellular Vesicles with Total Protein Content Assay, Nanoparticles Tracking Analysis, and Capillary Electrophoresis. Int J Mol Sci. 2022 Apr 1;23(8):4347. https://doi.org/10.3390/IJMS23084347/S1
8. Dziomba S, Wysocka M, Jońca J, Sola L, Steć A, Waleron K, et al. Investigation of selected parameters of capillary zone electrophoresis method for analysis of isolates of outer membrane vesicles. Electrophoresis. 2021 Oct 1;42(20):2010–7. https://doi.org/10.1002/ELPS.202000360
9. Jonca J, Waleron M, Czaplewska P, Bogucka A, Steć A, Dziomba S, et al. Membrane vesicles of Pectobacterium as an effective protein secretion system. Int J Mol Sci. 2021 Nov 1;22(22):12574. https://doi.org/10.3390/IJMS222212574/S1
10.Piotrowska M, Ciura K, Zalewska M, Dawid M, Correia B, Sawicka P, et al. Capillary zone electrophoresis of bacterial extracellular vesicles: A proof of concept. J Chromatogr A. 2020 Jun 21;1621:461047. https://doi.org/10.1016/J.CHROMA.2020.461047
CONTACT
Dr. Habil. Krzysztof Waleron, Assoc. Prof.
Department of Pharmaceutical Microbiology
Faculty of Pharmacy
Medical University of Gdańsk
Al. Gen. J. Hallera 107
80-416 Gdańsk
Phone number 58 349 19 72
www.microbio.gumed.edu.pl
Dr. Habil. Szymon Dziomba
Department of Toxicology
Faculty of Pharmacy
Medical University of Gdańsk
Al. Gen. J. Hallera 107
80-416 Gdańsk
Phone number 58 349 16 73
E-mail: szymon.dziomba@gumed.edu.pl