Hemostasis Research Laboratory
Department of Pharmaceutical Pathophysiology
Faculty of Pharmacy
Department of Clinical Biochemistry, Division of Molecular Medicine
Faculty of of Medicine
CORE FACILITY ACTIVITIES
The laboratory was established in cooperation between the staff of the Department of Pharmaceutical Pathophysiology and the Department of Clinical Biochemistry. Our scientific work includes experimental nanomedicine, platelet pharmacology, studies of platelet interaction with tumor cells, and the role of thrombocytes in the process of metastasis. We offer the possibility of performing experiments or providing substantive support for your ongoing projects. Our scientific activities involve the following methods: eukaryotic cell culture, including organoids; assessment of substance cytotoxicity; Western blot technique; PCR; ELISA; flow cytometry, fluorescence microscopy, light aggregometry. We also have experience in obtaining funding from external sources. We collaborate with research teams from home and abroad:
We collaborate with research teams from home and abroad:
- University of Saskatchewan College of Medicine, Saskatoon, Canada
- School of Pharmacy and Pharmaceutical Sciences and School of Medicine, Trinity Biomedical Sciences Institute, Trinity College Dublin, Ireland
- Department of Chemistry, University of Ioannina, Greece
- Department of Surgery Surgical Research, Friedrich-Alexander-Universität Erlangen-Nürnberg, Germany
- Laboratory of Organic Functional Materials Technology, Department of Chemistry, University of Warsaw
- Section of Electron Microscopy, Department of Biology, University of Gdańsk, Poland
- Department of Molecular Biochemistry, Faculty of Chemistry, University of Gdańsk
- Department of Environmental Technology, Faculty of Chemistry, University of Gdańsk
- Department of Biomedical Chemistry, Faculty of Chemistry, University of Gdańsk
- Department of Inorganic Chemistry, Faculty of Chemistry, University of Gdansk
- Department of Organic Chemistry, Department of Chemistry, Gdańsk University of Technology
- Department of Pharmacology, Medical University of Gdańsk
- Department of Applied Pharmacy, Medical University of Gdańsk
- Department of Pharmacognosy, Medical University of Gdańsk
- Department of Pharmaceutical Microbiology, Medical University of Gdańsk
- Department of Inorganic Chemistry, Medical University of Gdańsk
Division of Molecular Medicine
The Division of Molecular Medicine deals with analyses of pathomechanisms that underlie various diseases, at the molecular level. We are working on understanding the molecular basis of various disease entities, in vitro, ex vivo, as well as in vivo, therefore we have developed close cooperation with Colleagues from various Departments and Clinics of our University. Our scientific activities involve the following techniques: isolation of various types of nucleic acids from biological specimens; determination of polymorphisms, mutations of selected genes – real-time PCR; assessment of gene expression levels – real-time RT-PCR;qualitative and quantitative analysis of microRNAs – real-time RT-PCR; cell culture (cell lines and primary lines); cytotoxicity assessment of selected compounds: MTT, LDH, AP; protein assessment: ELISA, Western blot, immunofluorescence; overexpression or silencing of gene expression by shRNA using a lentivirus system. In terms of the foregoing molecular methods: we offer both substantive support and performing experiments.
Available equipment and devices
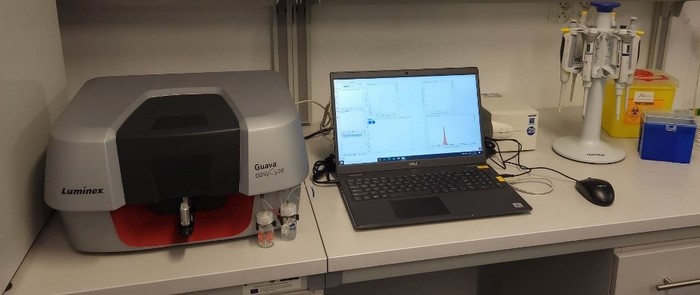
Guava easyCyte 5 Flow Cytometer – capillary flow cytometer that, using photomultiplier tubes (PMTs), allows detection in 3 channels of fluorescence and scattered light detection (FSC – front scattered light detector and SSC – side scattered light detector) to obtain information on the relative size of cells and the size and density of granules in the cytoplasm, respectively.
- excitation – blue laser: 488 nm (50 mW)
- detection green 525/30 nm; yellow: 583/26 nm; red: 695/50 nm
- minimum volume of the test sample- 150 µL in a tube
- minimum density of cells for analysis – 10 cells/µL
- ability to analyze cells in the size range of 0.4 -60 µm
- the system does not require the use of shielding fluids
- direct measurement of cell concentration in any sample volume, without the need for additional standard reagents
- software to manage the operating parameters of the device and analyze the obtained data

Diagnostic Fluorescence Inverted Microscope – MW 50 Series (MW50FL version)
- lenses:
- PH achromat plan grade for bright field and phase contrast 4x, 10x:
- 4X/0.13, WD10.4mm
- 10X/0.25, WD7.3mm
- semi plan apochromat PH grade, made from fluorite glass, for bright field and phase contrast 20X, 40X:
- 20X/0.45, WD5.91mm
- 40X/0.65, WD1.61mm
- semi plan apochromat grade, made from fluorite glass, for bright field 4x, 10x, 60x:
- 4X/0.13, WD18.52 mm
- 10X/0.30, WD7.11 mm
- 60X/0.75, WD1.04 mm
- three-place slide for phase contrast and bright field with space for filters, second
- two-place slide for using filters along with phase contrast, green and blue filter included
- fluorescence:
- HBO 100-W mercury lamp
- three filter blocks with B, G, UV filters
- universal camera dedicated to fluorescence – OPTA-TECH MI5FL with 5MPix resolution:
- pixel size: 3.45µm x 3.45µm
- live view speed: 35 fps for 5MP resolution – maximum photo resolution: 2448×2048
- “global shutter” – Exposure: from 0.13 ms. – 15s
- PH achromat plan grade for bright field and phase contrast 4x, 10x:
In addition, we have two fully equipped in vitro laboratories and the equipment necessary for protein analysis by Western blot, a multifunctional plate reader (measuring fluorescence, luminescence and absorbance), light aggregometers, the equipment needed for PCR reactions, and a UV-Vis spectrophotometer. We have experience in culturing primary and commercial cell lines, and also organoids as a 3D cell model. We have an established methodology for the isolation of cells of individual hematopoietic lineages from human peripheral blood. We have about 50 different cell lines in our biobank.

System PCR w czasie rzeczywistym LightCycler® 480 Roche
The LightCycler® 480 Instrument II is a high-speed, 96-well plate-based cycler with integrated real-time on-line detection capabilities. The special arrangement of optical elements in the LightCycler® 480 Instrument II ensures uniform collection of signals on the plate, and makes the analysis independent of sample position on the plate.
The LightCycler® 480 system configuration allows using all current probe formats (e.g., SYBR Green I, ResoLight high-resolution melt dye, hydrolysis probes, HybProbe probes, SimpleProbe probes), providing a solution for rapid and precise qualitative or quantitative detection of nucleic acids, genotyping and mutation scanning. Channels and filters can be combined in a variety of ways to provide flexibility for mono-color and multiplex testing. The pre-installed LightCycler® 480 software provides an intuitive, easy-to-use user interface for real-time PCR reaction programming, data storage and analysis. It facilitates highly comprehensive and extensive data analysis to study gene expression and genetic variation
BSL-2 microbiology laboratory
In addition, we have a separate microbiology lab, also with infrastructure for cell culture, where you can safely work with bacterial or viral systems for precisely increasing or silencing the expression of the studied genes.
PUBLICATIONS
Publications that have been developed by using the research methods described:
– Inkielewicz-Stepniak I., Tajber L., Behan G., Zhang H., Radomski M.W., Medina C., Santos-Martinez M.J. The Role of Mucin in the Toxicological Impact of Polystyrene Nanoparticles. Materials (Basel). 2018 May 3;11(5):724. doi: 10.3390/ma11050724.
– Medina C., Harmon S., Inkielewicz I., Santos-Martinez M.J., Jones M., Cantwell P., Bazou D., Ledwidge M., Radomski M.W., Gilmer J.F. Differential inhibition of tumour cell-induced platelet aggregation by the nicotinate aspirin prodrug (ST0702) and aspirin. Br J Pharmacol. 2012 Jun;166(3):938-49. doi: 10.1111/j.1476-5381.2011.01794.x.
– Hajtuch J., Santos-Martinez M.J., Wojcik M., Tomczyk E., Jaskiewicz M., Kamysz W., Narajczyk M., Inkielewicz-Stepniak I. Lipoic Acid-Coated Silver Nanoparticles: Biosafety Potential on the Vascular Microenvironment and Antibacterial Properties. Front Pharmacol. 2022 Jan 28;12:733743. doi: 10.3389/fphar.2021.733743.
– Hajtuch J., Iwicka E., Szczoczarz A., Flis D., Megiel E., Cieciórski P., Radomski M.W., Santos-Martinez M.J., Inkielewicz-Stepniak I. The Pharmacological Effects of Silver Nanoparticles Functionalized with Eptifibatide on Platelets and Endothelial Cells. Int J Nanomedicine. 2022 Sep 19;17:4383-4400. doi: 10.2147/IJN.S373691.
– Steckiewicz K.P., Adamska A., Narajczyk M., Megiel E., Inkielewicz-Stepniak I. Fluoride enhances polystyrene nanoparticles cytotoxicity in colonocytes in vitro model. Chem Biol Interact. 2022 Nov 1;367:110169. doi: 10.1016/j.cbi.2022.110169.
– SteckiewicZ K.P., Cieciórski P., Barcińska E., Jaśkiewicz M., Narajczyk M., Bauer M., Kamysz W., Megiel E., Inkielewicz-Stepniak I. Silver Nanoparticles as Chlorhexidine and Metronidazole Drug Delivery Platforms: Their Potential Use in Treating Periodontitis. Int J Nanomedicine. 2022 Feb; 17: 495–517. doi: 10.2147/IJN.S339046.
– Kowalski S., Wyrzykowski D., Hac S., Rychlowski M., Radomski M.W., Inkielewicz-Stepniak I. New Oxidovanadium(IV) Coordination Complex Containing 2-Methylnitrilotriacetate Ligands Induces Cell Cycle Arrest and Autophagy in Human Pancreatic Ductal Adenocarcinoma Cell Lines. Int J Mol Sci. 2019 Jan 10;20(2):261. doi: 10.3390/ijms20020261.
Division of Molecular Medicine:
– Kowalski R, Pikul P, Lewandowski K, Sakowicz-Burkiewicz M, Pawełczyk T, Zyśk M.: The cAMP Inducers Modify N-Acetylaspartate Metabolism in Wistar Rat Brain (Basel). 2021 Sep 1;10(9):1404. doi: 10.3390/antiox10091404.
– Starzyńska A, Sobocki BK, Sejda A, Sakowicz-Burkiewicz M, Szot O, Jereczek-Fossa BA.: ZNF-281 as the Potential Diagnostic Marker of Oral Squamous Cell Carcinoma. Cancers (Basel). 2021 May 28;13(11):2661. doi: 10.3390/cancers13112661.
– Chyła-Danił G, Sałaga-Zaleska K, Kreft E, Krzesińska A, Herman S, Kuchta A, Sakowicz-Burkiewicz M, Lenartowicz M, Jankowski M.: Suramin Affects the Renal VEGF-A/VEGFR Axis in Short-Term Streptozotocin-Induced Diabetes. Pharmaceuticals (Basel). 2023 Mar 22;16(3):470. doi: 10.3390/ph16030470.
– Zyśk M, Sakowicz-Burkiewicz M, Pikul P, Kowalski R, Michno A, Pawełczyk T.: The Impact of Acetyl-CoA and Aspartate Shortages on the N-Acetylaspartate Level in Different Models of Cholinergic Neurons. Antioxidants (Basel). 2020 Jun 13;9(6):522. doi: 10.3390/antiox9060522.
– Starzyńska A, Adamska P, Sejda A, Sakowicz-Burkiewicz M, Adamski ŁJ, Marvaso G, Wychowański P, Jereczek-Fossa BA.: Any Role of PIK3CA and PTEN Biomarkers in the Prognosis in Oral Squamous Cell Carcinoma? Life (Basel). 2020 Dec 3;10(12):325. doi: 10.3390/life10120325.
– Kreft E, Sałaga-Zaleska K, Sakowicz-Burkiewicz M, Dąbkowski K, Szczepánska-Konkel M, Jankowski M.: Diabetes Affects the A1 Adenosine Receptor-Dependent Action of Diadenosine Tetraphosphate (Ap4A) on Cortical and Medullary Renal Blood Flow. J Vasc Res. 2021;58(1):38-48. doi: 10.1159/000511461.
– Trzeciak M, Olszewska B, Sakowicz-Burkiewicz M, Sokołowska-Wojdyło M, Jankau J, Nowicki RJ, Pawełczyk T.: Expression Profiles of Genes Encoding Cornified Envelope Proteins in Atopic Dermatitis and Cutaneous T-Cell Lymphomas. Nutrients. 2020 Mar 24;12(3):862. doi: 10.3390/nu12030862.
– Starzyńska A, Sobocki BK, Sakowicz-Burkiewicz M, Jereczek-Fossa BA, Alterio D, Szot O, Korwat A, Pęksa R.: VISTA H-Score Is Significantly Associated with a 5-Year DFS Rate in Oral Squamous Cell Carcinoma. J Clin Med. 2023 Feb 17;12(4):1619. doi: 10.3390/jcm12041619.
– Zyśk M, Pikul P, Kowalski R, Lewandowski K, Sakowicz-Burkiewicz M, Pawełczyk T.: Neither Excessive Nitric Oxide Accumulation nor Acute Hyperglycemia Affects the N-Acetylaspartate Network in Wistar Rat Brain Cells. Int J Mol Sci. 2020 Nov 12;21(22):8541. doi: 10.3390/ijms21228541.
– Zyśk M, Gapys B, Ronowska A, Gul-Hinc S, Erlandsson A, Iwanicki A, Sakowicz-Burkiewicz M, Szutowicz A, Bielarczyk H.: Protective effects of voltage-gated calcium channel antagonists against zinc toxicity in SN56 neuroblastoma cholinergic cells. PLoS One. 2018 Dec 20;13(12):e0209363. doi: 10.1371/journal.pone.0209363. eCollection 2018.
– Adamski ŁJ, Starzyńska A, Adamska P, Kunc M, Sakowicz-Burkiewicz M, Marvaso G, Alterio D, Korwat A, Jereczek-Fossa BA, Pęksa R.: High PD-L1 Expression on Tumor Cells Indicates Worse Overall Survival in Advanced Oral Squamous Cell Carcinomas of the Tongue and the Floor of the Mouth but Not in Other Oral Compartments. Biomedicines. 2021 Sep 1;9(9):1132. doi: 10.3390/biomedicines9091132.
– Czaplińska M, Ćwiklińska A, Sakowicz-Burkiewicz M, Wieczorek E, Kuchta A, Kowalski R, Kortas-Stempak B, Dębska-Ślizień A, Jankowski M, Król E.: Apolipoprotein E gene polymorphism and renal function are associated with apolipoprotein E concentration in patients with chronic kidney disease. Lipids Health Dis. 2019 Mar 9;18(1):60. doi: 10.1186/s12944-019-1003-x.
PRICE LIST
Cooperation with our Unit is possible on a commercial basis or as a part of scientific and research projects. Pricing for the performance of experiments will be provided individually for each project.
CONTACT

Prof. Iwona Inkielewicz-Stępniak
Department of Pharmaceutical Pathophysiology
Faculty of Pharmacy
Dębinki 7 St., Building #27
80-211 Gdańsk
Phone 58 349 15 16
Mail iwona.inkielewicz-stepniak@gumed.edu.pl

Prof. Monika Sakowicz-Burkiewicz
Division of Molecular Medicine
Department of Clinical Biochemistry
Faculty of of Medicine
Dębinki 7 St., Building #27
80-211 Gdańsk
Phone 58 349 27 59
Mail monika.sakowicz-burkiewicz@gumed.edu.pl
photo Paweł Sudara/MUG & private archive

