Laboratory of Toxicological Analytics
Department of Toxicology
Faculty of Pharmacy
ABOUT US
The Laboratory of Toxicological Analytics at the Department of Toxicology (Faculty of Pharmacy, Medical University of Gdańsk) specializes in trace analysis of xenobiotics in biological specimens and environmental samples. Thanks to numerous years of experience, the unit’s personnel can offer substantive support to MUG’s doctoral students and employees, as well as to external institutions, as a part of ongoing research and projects.
OUR POTENTIAL
We offer cooperation in: development, optimization and validation of analytical methods for qualitative and quantitative analysis of xenobiotics (medicinal substances, drugs, alcohols, pesticides) and their metabolites in biological specimens (biomonitoring) and environmental samples.
The analytical procedures used in the laboratory are subject to routine intra- and extra-laboratory inspections (participation in the German External Quality Assessment Scheme (G-EQUAS) program and the HBM4EU project).
- Biological matrices: urine, blood, follicular fluid, hair, saliva
- The list of analyzed substances includes:
- bisphenol A and its structural analogs, parabens and other endocrine disruptors,
- metabolites of synthetic pyrethroids,
- fipronil
- Instrumental techniques used: GC-MS/MS, LC-MS/MS
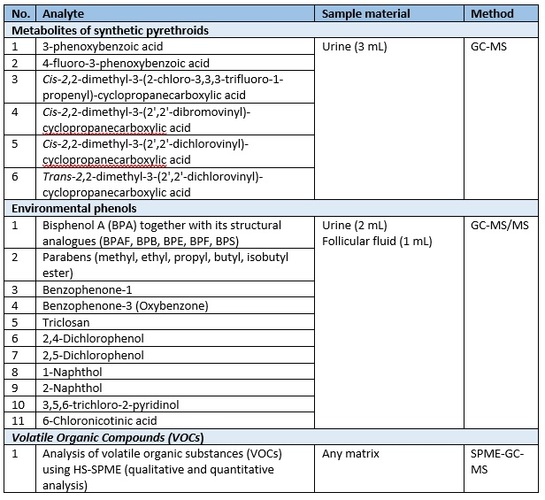
Table 1. List of available analytical methods for biological monitoring.
SCOPE OF SERVICES
- optimization and validation of extraction methods (including SPE, LLE, head-space)
- optimization of chromatographic separation conditions (GC and LC)
- optimization of MS and MS/MS detection conditions (GC and LC)
- pharmaceutical analysis
- analysis of selected xenobiotics and their metabolites in biological samples
- toxicokinetics testing for xenobiotics
- in chemico biotransformation tests using an electrochemical cell (ROXY EC System, Antec)
- estimating the magnitude of exposure based on measuring the concentration of exposure biomarkers in biological specimens
- quality control of extracellular vesicle isolates using capillary electrophoresis. The technique can also be used for quality control of pharmaceutical and biotechnology products, in chiral analysis and drug purity testing. It is primarily dedicated to the separation of hydrophilic substances. The system is adapted to implement basic electromigration techniques (CZE, MEKC, ITP, cIEF, CGE).
AVAILABLE EQUIPMENT
- Gas chromatograph (Varian 3800) coupled to a triple quadrupole mass spectrometer (Varian 320ms)

- Gas chromatograph (Varian 450) coupled to a quadrupole ion trap mass spectrometer (Varian 220ms)

- Gas chromatograph (Scion 436) coupled to a flame ionization detector (FID) and an attachment for the analysis of the supersurface phase

- Gas chromatograph (Bruker 456) coupled to an electron capture detector (ECD)

- Liquid chromatograph (Varian) coupled to a triple quadrupole mass spectrometer (Varian 320ms)

- Liquid chromatographs with detectors: DAD, UV/Vis, fluorescence, electrochemical
- Capillary electrophoresis with DAD and LIF detector
PRICE LIST
The laboratory performs research and analysis according to individual projects. Service costs are valued individually.
PUBLICATIONS
Many publications have been developed based on the results of the research obtained by using the knowledge, experience of scientists from the Department and its equipment, including.
1. Steć A, Chodkowska M, Kasprzyk-Pochopień J, Mielczarek P, Piekoszewski W, Lewczuk B, Płoska A, Kalinowski L, Wielgomas B, Dziomba S. Isolation of Citrus lemon extracellular vesicles: Development and process control using capillary electrophoresis. Food Chem. 2023 May 12;424:136333. doi: 10.1016/j.foodchem.2023.136333. Epub ahead of print. PMID: 37201469.
2. Klimowska A, Wynendaele E, Wielgomas B. Quantification and stability assessment of urinary phenolic and acidic biomarkers of non-persistent chemicals using the SPE-GC/MS/MS method. Anal Bioanal Chem. 2023 May;415(12):2227-2238. doi: 10.1007/s00216-023-04633-7. Epub 2023 Mar 18. PMID: 36933054; PMCID: PMC10115689.
3. Radwan P, Wielgomas B, Radwan M, Krasiński R, Bujak-Pietrek S, Polańska K, Kilanowicz A, Jurewicz J. Urinary concentration of selected nonpersistent endocrine disrupting chemicals-reproductive outcomes among women from a fertility clinic. Environ Sci Pollut Res Int. 2023 Mar;30(15):45088-45096. doi: 10.1007/s11356-023-25355-4. Epub 2023 Jan 26. PMID: 36701050; PMCID: PMC10076394.
4. Radwan P, Wielgomas B, Radwan M, Krasiński R, Kilanowicz-Sapota A, Banaszczyk R, Jurewicz J. Synthetic Pyrethroids Exposure and Embryological Outcomes: A Cohort Study in Women from Fertility Clinic. Int J Environ Res Public Health. 2022 Apr 22;19(9):5117. doi: 10.3390/ijerph19095117. PMID: 35564520; PMCID: PMC9100335.
5. Rodzaj W, Wileńska M, Klimowska A, Dziewirska E, Jurewicz J, Walczak-Jędrzejowska R, Słowikowska-Hilczer J, Hanke W, Wielgomas B. Concentrations of urinary biomarkers and predictors of exposure to pyrethroid insecticides in young, Polish, urban-dwelling men. Sci Total Environ. 2021 Jun 15;773:145666. doi: 10.1016/j.scitotenv.2021.145666. Epub 2021 Feb 6. PMID: 33596511.
6. Radwan P, Wielgomas B, Radwan M, Krasiński R, Klimowska A, Zajdel R, Kaleta D, Jurewicz J. Triclosan exposure and in vitro fertilization treatment outcomes in women undergoing in vitro fertilization. Environ Sci Pollut Res Int. 2021 Mar;28(10):12993-12999. doi: 10.1007/s11356-020-11287-w. Epub 2020 Oct 23. PMID: 33097990; PMCID: PMC7921062.
7. Klimowska A, Amenda K, Rodzaj W, Wileńska M, Jurewicz J, Wielgomas B. Evaluation of 1-year urinary excretion of eight metabolites of synthetic pyrethroids, chlorpyrifos, and neonicotinoids. Environ Int. 2020 Dec;145:106119. doi: 10.1016/j.envint.2020.106119. Epub 2020 Sep 17. PMID: 32950790.
8. Jurewicz J, Radwan P, Wielgomas B, Radwan M, Karwacka A, Kałużny P, Piskunowicz M, Dziewirska E, Hanke W. Exposure to pyrethroid pesticides and ovarian reserve. Environ Int. 2020 Nov;144:106028. doi: 10.1016/j.envint.2020.106028. Epub 2020 Aug 11. PMID: 32795752.
9. Krenczkowska D, Mojsiewicz-Pieńkowska K, Wielgomas B, Bazar D, Jankowski Z. Ex Vivo Human Skin is not a Barrier for Cyclic Siloxanes (Cyclic Silicones): Evidence of Diffusion, Bioaccumulation, and Risk of Dermal Absorption Using a New Validated GC-FID Procedure. Pharmaceutics. 2020 Jun 24;12(6):586. doi: 10.3390/pharmaceutics12060586. PMID: 32599732; PMCID: PMC7355424.
10. Piotrowska M, Ciura K, Zalewska M, Dawid M, Correia B, Sawicka P, Lewczuk B, Kasprzyk J, Sola L, Piekoszewski W, Wielgomas B, Waleron K, Dziomba S. Capillary zone electrophoresis of bacterial extracellular vesicles: A proof of concept. J Chromatogr A. 2020 Jun 21;1621:461047. doi: 10.1016/j.chroma.2020.461047. Epub 2020 Mar 13. PMID: 32197757.
11. Klimowska A, Wielgomas B. Off-line microextraction by packed sorbent combined with on solid support derivatization and GC-MS: Application for the analysis of five pyrethroid metabolites in urine samples. Talanta. 2018 Jan 1;176:165-171. doi: 10.1016/j.talanta.2017.08.011. Epub 2017 Aug 9. PMID: 28917736.
CONTACT
Michał May, Ph.D.
michal.may@gumed.edu.pl
Laboratory of Toxicological Analytics
at the Department of Toxicology
Faculty of Pharmacy
Medical University of Gdańsk
al. Gen. J. Hallera 107
80-416 Gdańsk
Phone 58 349 16 75
photo Paweł Sudara/MUG