Cytogenetic and Molecular Diagnostics Laboratory
Department of Biology and Medical Genetics
Faculty of Medicine
Medical University of Gdańsk
As part of the “Excellence Initiative – Research University” program, the Cytogenetic and Molecular Diagnostics Laboratory at the MUG is expanding its activities. The unit has performed cytogenetic and molecular testing, both cancer and non-cancer, for over 25 years for public and non-public healthcare facilities, hospitals, private practices, and individuals. In 2012, the laboratory was entered on the list of medical laboratories of the National Chamber of Laboratory Diagnosticians. The tests we performed were a healthcare service to prevent, preserve, save, restore and improve health. Currently, the goal of the Laboratory will also be the scientific activity of conducting and assisting in molecular analyses. The research team includes experienced laboratory diagnosticians, also with a specialty in laboratory medical genetics.
Our potential
1. Ultrasonifier for DNA fragmentation
2. Automated system for the isolation of nucleic acids
3. Genomic sequencer
photo: Monika Żuk, Ph.D. /MUG
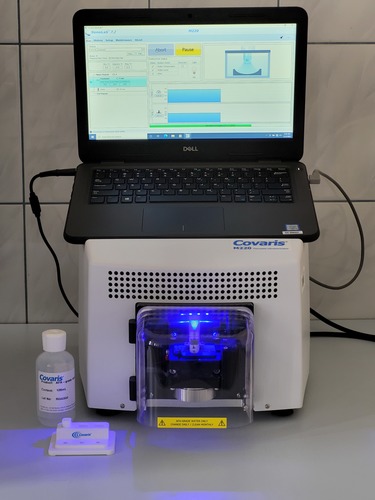
Ultrasonifier for DNA fragmentation
Maxwell® CSC (Clinical Sample Concentrator) is designed specifically for in vitro analysis in molecular diagnostics. Manufactured according to GMP guidelines, the device is used to isolate high quality nucleic acids and guarantees reproducible results using appropriate CE IVD certified kits.
Application:
- Sample preparation for next generation sequencing (NGS)
- Extraction of DNA/RNA from Formalin-Fixed Paraffin-Embedded (FFPE) samples
- Extraction of DNA from blood plasma
- DNA extraction for NGS analysis from whole blood
- DNA extraction from dry blood drop (DBS)
- Biomarker extraction
- Low-mass chromatin fragmentation
Generation of DNA fragments between 150 bp and 5000 bp
Use of technology that allows focused energy to be directed at a specific volume of sample
Working with sample volumes in the range of 15 µL-130 µL
An external control station coupled to the system:
1. A laptop with specifications that ensure seamless system operation.
2. Dedicated control software:
- Protocols for creating DNA fragments in the range of 150 to 5000 bp * Ability to generate arbitrary DNA fragment creation protocols
- Real-time process monitoring
3. Installed operating system compatible with the software
Automated system for the isolation of nucleic acids
Covaris M220 is designed to prepare samples for Next Generation Sequencing (NGS) and can generate DNA fragments in the range of 150 bp – 5000 bp. The device works with a variety of DNA fragmentation protocols based on Covaris SonoLab™ software and uses Covaris microTUBE and miniTUBE accessories. Built-in temperature control eliminates the need to purchase an external cooling system. Covaris AFA® (Adaptive Focused Acoustics®) technology allows energy to be focused on a specific area of the sample and gives control over the length of the DNA fragments generated. The fragmentation process itself is quiet, isothermal and non-contact.
Covaris M220 DNA fragmentation apparatus for sequencing applications Next Generation Genetics (NGS):
1. DNA fragmentation process based on patented AFA technology (Adaptive Focused Acoustics®)
2. a technique recognized as the “gold standard” used worldwide in genetic laboratories
3. generation of highly reproducible DNA fragment distributions
4. developed protocols for creating fragments in the range of 150 to 5000 bp
5. ability to fragment low mass chromatin with high recovery of epitopes
6. built-in cooling system
7. start-up time of less than one minute
8. precise and standardized results
9. elimination of variability of results caused by user action
10. real-time process monitoring
11. internal quality control in the Covaris SonoLab™ program
12. NIST-calibrated instrument
Application:
In vitro diagnostic (IVD) medical device:
- Isolation of nucleic acids from peripheral blood
- Isolation of nucleic acids from Formalin-Fixed Paraffin-Embedded
- Tissues (FFPET)
Research Objectives (RUO):
- Isolation of nucleic acids from peripheral blood plasma
- Isolation of nucleic acids from tissue homogenate
- Isolation of nucleic acids from swab samples
Isolation technique based on the interaction of magnetic microparticles with DNA/RNA, with bead transport within the cartridge being unidirectional and without liquid transfer
Possibility of using the isolated material for further molecular analyses
Ability to isolate at least 16 nucleic acid samples in 20-60 minutes
Ability to isolate at least 16 nucleic acid samples in 20-60 minutes
System operation coupled to dedicated reagent kits – isolation DNA/RNA in IVD or RUO mode
Activation of the isolation protocol by scanning the barcode of a set of dedicated reagents
Possibility to set different elution volumes for individual samples
System control via a graphical interface on the tablet
Tablet suitable for use with laboratory gloves
A tablet with specifications that guarantee seamless system operation:
1. Dedicated control software:
- Pre-programmed IVD mode protocols
- Pre-programmed RUO mode protocols
- Ability to generate arbitrary isolation protocols
- Report generation
Genomic sequencer
MiSeq uses Illuminy’s proven sequencing-by-synthesis technology. It allows obtaining approximately 13 GB (13×109 bases) in 45 million paired reads from a single sequencing.
The amount of this data enables:
- sequencing of small genomes (bacteria, yeast, viruses) or plasmids
- amplicon sequencing
- sequencing of small RNA, and bacterial transcriptomes
- sequencing of a 16S RNA coding fragment or ITS fragments from a pool of environmental DNA (metagenomics), allowing determination of population composition.
The next-generation MiSeq sequencer is a desktop system using SBS – sequencing by synthesis – technology. The MiSeq integrates the steps of amplification, sequencing, and data analysis into a single compact device. The instrument takes readings up to 2×300 bp and generates up to 15 Gb of product during a single operation cycle. Ease of use, high accuracy of readings and low price make this platform an ideal tool for quick and economic genetic analysis. The MiSeq enables a wide range of applications such as targeted sequencing, metagenomics, small genome sequencing, targeted gene expression, amplicon sequencing, and HLA analysis.
The resulting data can be processed locally on the embedded computer or exported and analyzed using a wide set of programs in the BaseSpace distributed environment. The instrument is also available in a medical diagnostic version, the MiSeq Dx, and a dedicated forensic genetics version, the Miseq FGx.
RESEARCH TEAM
The research team includes:
- Prof. Bartosz Wasąg
- Dr. Habil. Magdalena Ratajska
- Alina Kuźniacka, Ph.D.
- Monika Żuk, Ph.D.
- Monika Targońska, M.A.
- Katarzyna Wojciechowska, M.A.
PUBLICATIONS
Publications based on research results obtained using our research equipment:
ALK alterations in salivary gland carcinomas. Majewska H, Gorczyński A Czapiewski P, Menon R, Mueller J, Lakis S, Heuckmann JM, Loco J, Gupta R, Andreasen S, Stodulski D, lliszko M, Dziadziuszko R, Jassem J, Heukamp LC, Biernat W. Virchows Arch. 2020 Nov 25. doi: 10.1007 / s00428-020-02971
Expression of Female Sex Hormone Receptors, Connective Tissue Growth Factor and HER2 in Gallbladder Cancer. Hryciuk B, Pęksa R. Bieńkowski M, Szymanowski B, Radecka B, Winnik K, Żok J, Cichowska N, lliszko M , Duchnowska R. Sci Rep. 2020 Feb 5;10(1 ):1871. doi: 10.1038/s41598-020-58777-y
MAML2 rearrangement as a useful diagnostic marker discriminating between Warthin tumour and Warthin-like mucoepidermoid carcinoma. Bieńkowski M, Kunc M, lliszko M, Kuźniacka A Studniarek M, Biernat W. Virchows Arch. 2020 Sep;477(3):393-400. doi: l 0.1007 /s00428-020-02798-5
Colorectal Adenocarcinomas Harboring ALK Fusion Genes: A Clinicopathologic and Molecular Genetic Study of 12 Cases and Review of the Literature. Lasota J, Chłopek M, Wasąg B, Kowalik A, Christiansen J, Lamoureux J, Kuźniacka A, Felisiak-Gołąbek A, Liu Y, Reyes TAR, Saha R, Agaimy A, Behenska K, Biernat W, Cattaneo L, Centonze G, Daum O, Daumova M, Domagała P,Dziuba I, Geppert CE, Góźdź S, Nasierowska-Guttmejer A, Hałoń A, Hartmann A lnaguma S, lżycka-Świeszewska E, Kaczorowski M, Kołos M, Kopczyński J, Michal M, Milione M, Okoń K, Pęksa R, Pyzlak M, Ryś J, Waloszczyk P, Wejman J, Miettinen M. Am J Surg Pathol. 2020 Sep;44(9):1224-1234. doi: 10.1097/ PAS. 0000000000001 51 2
Genetic Mosaicism in a Group of Patients With Cornelia de Lange Syndrome. Krawczynska N, Wierzba J, Wasag B. Front Pediatr. 2019 May 15;7:203. doi: l 0.3389/fped.2019.00203.
New Approach to Paediatric Mastocytosis: Implications of KIT D816V Mutation Detection in Peripheral Blood. Czarny J, Żuk M, Zawrocki A, Plata-Nazar K, Biernat W, Niedoszytko M, Ługowska-Umer H, Nedoszytko B, Wasąg B, Nowicki RJ, Lange M. Acta Derm Venereol. 2020 May 28;100{10):adv00149. doi: 10.2340/00015555-3504
Afatinib in NSCLC With HER2 Mutations: Results of the Prospective, Open-Label Phase II NICHE Trial of European Thoracic Oncology Platform {ETOP). Dziadziuszko R, Smit EF, Dafni U, Wolf J, Wasąg B, Biernat W, Finn SP, Kammler R, Tsourti Z, Rabaglio M, Ruepp B, Roschitzki-Voser H, Stahel RA, Felip E, Peters S. J Thorac Oncol. 2019 Jun;l4{6):1086-1094. doi: 10.1016/j.jtho.2019.02.017.
Haplotypes of butyrylcholinesterase K-variant and their influence on the enzyme activity. Jasiecki J, Żuk M, Krawczyńska N, Jońca J, Szczoczarz A, Lewandowski K, Waleron K, Wasąg B. Chem Biol Interact. 2019 Jul 1; 307:1 54-1 57. doi: 10.101 6/ j.c b i.20 1 9.05.007. Epub 2019 May 6. PMID: 31071335
Synergy between the alteration in the N-terminal region of butyrylcholinesterase K variant and apolipoprotein E4 in late-onset Alzheimer’s disease. Jasiecki J, Limon-Sztencel A, Żuk M, Chmara M, Cysewski D, Limon J, Wasąg B. Sci Rep. 2019 Mar 26;9{1 ):5223. doi: 10.1038/s4 l 598-0l 9-4l 578-3. PMID: 30914707
PRINCE LIST
The laboratory will perform analyses according to individual orders with adaptation to specific needs of contractors on the basis of service activity or project participation.
CONTACT
Monika Żuk, Ph.D.
Department of Biology and Medical Genetics
Medical University of Gdańsk
Dębinki 1 Street
80-211 Gdańsk
Phone: 58 349 15 32
monika.zuk@gumed.edu.pl